Bio-diesel from Ethanol
One of the fastest growing source of biodiesel is inedible corn oil produced as a byproduct of corn ethanol. Corn oil has historically been more expensive than soybean oil, and thus not an attractive source of biodiesel. But over the last few years, a new source of corn oil emerged that was competitively priced.
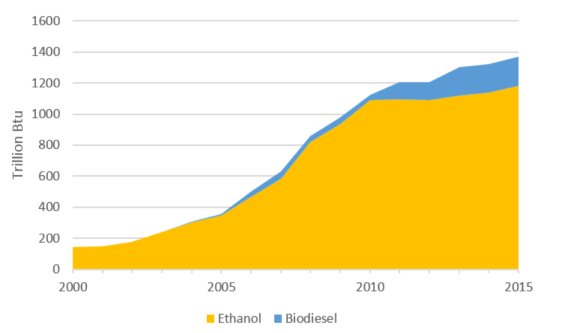
The corn ethanol boom of 2005 to 2010 saw a huge increase in production of distillers’ grains, an animal feed co-product of ethanol production that is left behind once the corn starch is made into ethanol. Ethanol producers learned that they could extract corn oil from the distillers’ grains, reducing the fat content of the animal feed in the process.
Biodiesel produced from distillers’ corn oil grew by about ten times from 2010 to 2013, but leveled off thereafter. Corn oil associated with distillers grains is limited by corn ethanol production, and while some further shifting of oil from feed to fuel markets is possible, the increase associated with the ethanol boom is unlikely to be repeated, and is not the basis for a sustainable trend into the future.
The Process
Biodiesel is a diesel fuel that is made by reacting vegetable oil (cooking oil) with other common chemicals. Biodiesel may be used in any diesel automotive engine in its pure form or blended with petroleum-based diesel. No modifications are required, and the result is a less-expensive, renewable, clean-burning fuel.
To date, corn oil has not been considered a viable biodiesel feedstock because of its high edible value and relatively high price, but some industrial corn processing co-products, such as corn germ and dried distillers grains with solubles (DDGS), have potential for this application after the extraction of corn distillers oil (CDO).
Transesterification
Transesterification consists of a reaction between an alcohol and vegetable oil or animal fat. The alcohol must preferably have a low molecular weight (such as methanol and ethanol) so that the reaction proceeds faster. An excess of alcohol is needed to shift the reaction equilibrium in the direction of product formation. The reaction takes place in the range of 20-60 oC with and alcohol to oil ratio of 6:1 to 12:1. Less than 1% of catalyst is required and the products include methyl or ethyl esters and glycerol.
Purification
After the reaction of transesterification, there is the separation of glycerol and ethyl or methyl esters, which are only called biodiesel after reaching the appropriate specifications, that is, after the removal of contaminants such as free glycerol, soaps, metals, excess alcohol, catalyst, and others.
